Abstract
Background: R-ISS, which includes ISS combined with presence of t(4;14), 17pdel or t(14;16) and/or elevated serum LDH has been proposed and validated in Europe. Early relapse (<24 months) is a strong predictor of worse MM outcomes after AHCT. We hypothesized that R-ISS at diagnosis will predict early relapse and remain prognostically important among early relapsers. We analyzed post-relapse overall survival (OS) by R-ISS among early post-AHCT MM relapsers.
Patients and Methods: Using the Center for International Blood and Marrow Transplant Research database, we identified MM patients receiving first AHCT in 2008-2014 with melphalan conditioning after novel therapy induction, within 18 months of diagnosis, and available diagnostic LDH and molecular studies (n= 628). Relative risk of relapse/progression, progression-free survival (PFS) and OS was calculated using the Cox proportional hazards regression with R-ISS group as predictor. A landmark analysis was conducted at 24 months to identify patients who relapsed within 24 months. Post-early relapse survival was tested in multivariate analysis to identify factors affecting post-relapse OS.
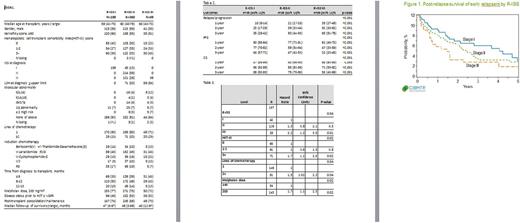
Results: The number of patients were: R-ISS stage I, N=199; II, N=360 and III, N=69. Baseline characteristics are shown in table 1. R-ISS II had 13% ISS I, 59% ISS II and 28% ISS III patients. More patients in R-ISS III had received more than 1 line of chemotherapy (29% compared to 20% for R-ISS II and 15% for R-ISS I). Disease status at AHCT was similar in all 3 groups. Worse relapse/progression, progression-free and overall survival was seen with higher R-ISS (table 2). The cumulative incidence of early relapse was 23% (R-ISS I), 39% (R-ISS II) and 50% (R-ISS III) groups (p <0.001). Multivariate analysis of post- relapse OS among early relapsers showed that R-ISS III at diagnosis was independently prognostic (table 3). Figure 1 shows post-relapse survival by R-ISS groups.
Conclusions: We conclude that R-ISS at diagnosis is predictive of early relapse after upfront autoHCT and R-ISS is independently prognostic of post-relapse survival among early relapsers after AHCT. Among patients who relapse early, HCT-CI≥3, use of >1 line of induction chemotherapy and melphalan dose are independently associated with worse post-relapse survival.
D'Souza: Celgene: Consultancy; Prothena: Research Funding; Merck: Research Funding. Gasparetto: Janssen, BMS, Celgene: Consultancy; Celgene: Research Funding; Janssen, BMS, Celgene: Other: Travel, accommodations, or other expenses paid or reimbursed; Janssen, BMS, Celgene, Takeda: Honoraria. Kumar: Skyline: Honoraria; Celgene, Millennium/Takeda, Onyx, AbbVie, Janssen, Sanofi, Novartis, Amgen, Genentech, Merck, Oncopeptides, Roche, Skyline Diagnostics: Research Funding; Celgene, Millennium, BMS, Onyx, Janssen, Noxxon, AbbVie, Amgen, Merck, Oncopeptides, Skyline Diagnostics, Takeda: Consultancy. Hari: Celgene: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.